One strange quantum rule is superposition. For example, when one photon goes through two slits at the same time in the double-slit experiment, it does so in a superposition. Solving an equation usually gives one solution that satisfies its conditions, but solving a quantum equation gives a set of solutions, where each is the probability that a physical event will occur. These solutions evolve over time, as the wave spreads, but at any moment only one of them can actually happen.
Quantum mathematics also has the strange feature that for any two solutions, their combination is also a solution, called a superposition (Note 1). Yet while single solutions match familiar physical events, these combined solutions never physically occur, so quantum states superpose in ways that physical states can’t. For example, in Young’s experiment, the photon goes through both slits at the same time in a superposed state, but we never observe a photon in both slits at once. This ability to superpose underlies the mysterious efficacy of quantum theory.
molecule states
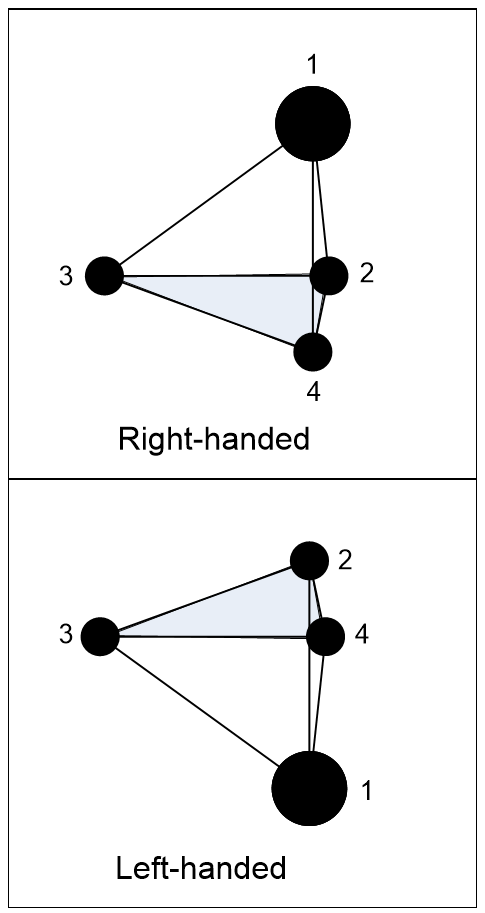
Superposition applies not only to photons but also to molecules. For example, ammonia molecules have a pyramid shape (Figure 3.20) with a nitrogen atom apex (1) and a base of hydrogen atoms (2, 3, 4). This molecule can occur in right or left-handed forms, but to turn a right-handed molecule into a left-handed one, a nitrogen atom must pass through the pyramid base, which is physically impossible (Feynman et al., 1977) III, p9-1. Yet according to quantum theory, both these states are valid solutions and so is their combination, so in the quantum world, an ammonia molecule can be in a right and left-handed superposition!
This explains the otherwise inexplicable finding that an ammonia molecule can be left-handed one moment and right-handed the next, yet it can’t physically change between these states. That the ammonia molecule is a left and right-handed superposition lets us observe either one, just as a photon superposed between two slits can be observed in either one.
To think that superposition is just ignorance of a hidden physical state is to misunderstand it, as superposed quantum currents can flow both ways round a superconducting ring at once but physical currents would cancel (Cho, 2000). As Young’s experiment shows, the superposed photon really does go through both slits at once. Superposition is physically impossible but it is just business as usual in the quantum world.
Superposition occurs because on a network, processing spreads in every possible way regardless of physical laws, so when a photon spreads through two slits, it literally half-exists in both. The photon process can spread itself around in ways that a photon particle can’t.
Why then don’t these combinations occur physically? It isn’t possible because a physical event is a processing restart that occurs at one point. Restarting a computer stops anything else it is doing and the same is true for a quantum process, so while an ammonia molecule can be in two quantum states at once, it can only restart from one of them. Superposed quantum states never occur physically because a physical event restarts one or the other, not both. Even so, we struggle to imagine how one entity can exist in incompatible ways at the same time, as Schrödinger’s cat illustrates.
Note 1. If Y1 and Y2 are state solutions of Schrödinger’s equation then (Y1 + Y2) is also a valid solution.