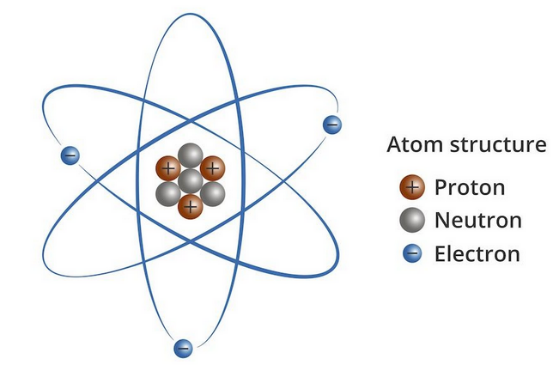
 In 1909, Rutherford described the atom as a nucleus around which electrons orbit as planets do the sun, but while planets occasionally collide, electrons never do. A lead atom has 82 electrons whizzing around in close proximity but is stable for billions of years, so why don’t they collide?
In 1909, Rutherford described the atom as a nucleus around which electrons orbit as planets do the sun, but while planets occasionally collide, electrons never do. A lead atom has 82 electrons whizzing around in close proximity but is stable for billions of years, so why don’t they collide?
A particle in orbit is also accelerating, so it should lose energy and spiral inwards, but again electrons never do, so why don’t the laws of physics apply in atoms? The standard model says virtual photons shield electrons from the nuclear attraction but what then keeps them in orbit? It also invented the miracle of wave-particle duality, that lets electrons be particles in space but waves in atoms. Physicists know that a particle isn’t a wave, and wave isn’t a particle, but this lets them choose the right equation. But how does the electron know to be a particle in space but a wave in the atom?
Apparently, electrons know Pauli’s exclusion principle, that they can overlap like waves if they have different quantum numbers. Quantum numbers were invented after the fact, to let electrons co-exist in atomic orbits, but they aren’t based on or compatible with any physical property.
However, electrons as one-dimensional matter can be like matter on one dimension but like light on the other two. Their matter dimension is why electrons in space move slower than light and collide like particles, but on the two-dimensional surface of an atomic orbit, they can be entirely wavelike.
The miracle of wave-particle duality then isn’t a miracle at all, but based on the matter-light duality of electrons. A particle orbiting a center needs an agent to stop it falling in but a wave on a circumference that fits its wavelength can pulse forever. Electrons then never collide in an atom because they are waves vibrating at different.
Electrons are particles in space but waves in atoms because they are one-dimensional matter.